A symposium proposal for ISOT2016 has been accepted with the draft title “Mechanisms of Background Segregation and Source Localization of Odors”.
August 2015
A symposium proposal for ISOT2016 has been accepted with the draft title “Mechanisms of Background Segregation and Source Localization of Odors”.
August 2015
Most of the project met in Konstanz for a first detailed planning meeting. A mini-sympoium was also held for the staff and students of the biology and neurobiology laboratories where project memebers presnted and discussed their past and proposed research.
July 2015
Other websites and online resources of interest:
Human Frontier Science Program (www.hfsp.org)
The Green Brain Project (www.greenbrain.group.shef.ac.uk)
The Brains on Board Project (EPSRC grants on the Web entry)
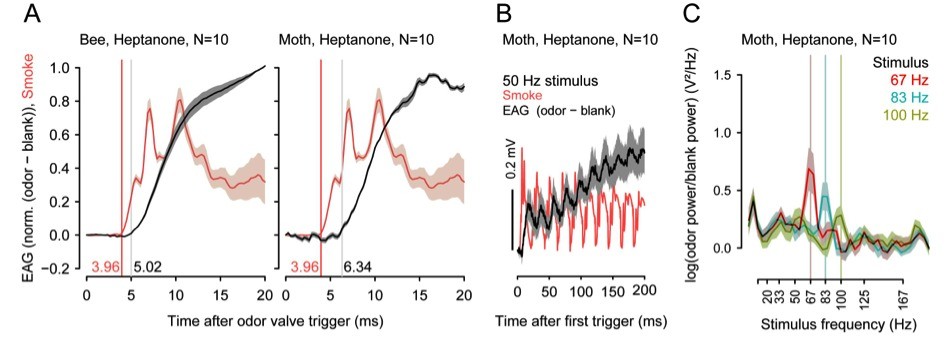
Insects can segregate concurrent odors from closely spaced sources based on short differences in the arrival of odorants. In preliminary experiments with different insect species, we found that odor transduction can occur within 1 to 3ms and odorant fluctuations can be resolved at frequencies above 100 Hz.
Thus, the insect brain could exploit fast stimulus dynamics for odor-background segregation, but how it accomplishes this segregation is not yet known. Moreover, learning modifies odor representations such that relevant odors become more salient, whereas less relevant odors are suppressed. This effect of odor learning might also be used to enhance odor-background segregation by making the AL more sensitive to particular odors.
It is our hypothesis, to be tested with this proposal, that bees use high temporal resolution and odor-learning for odor-background segregation. This allows the animal to create a neural representation of the spatio-temporal structure of the olfactory world. Odorants from the same sources fluctuate synchronously, while odorants from different sources fluctuate asynchronously. Understanding how small and fast fluctuations in relative concentrations of odor mixtures are used by animals to reliably identify odor sources will help us identify common principles of how animals represent and process their environment.
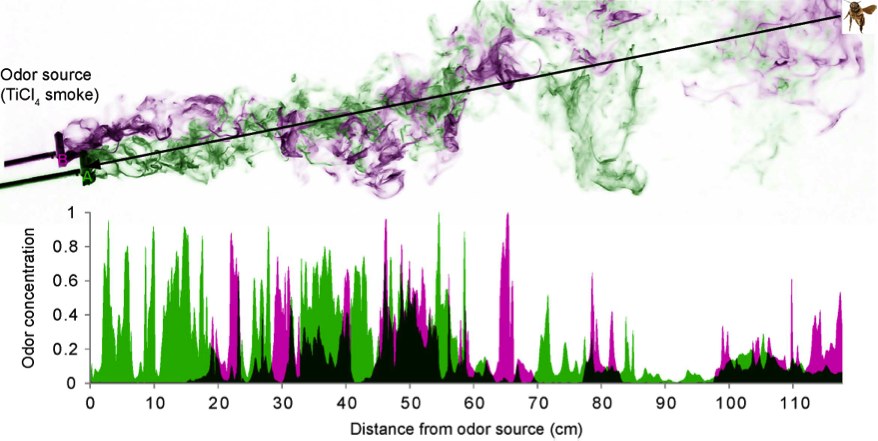
Odors occur in turbulent plumes and quickly break into thin filaments that intermingle with background odors.
We will measure the temporal structure of natural plumes of multiple odorants originating from separate sources with a flying vehicle equipped with moth antennae. We will use this information to mimic the temporal structure of natural multi-odorant plumes in behavioral and physiological experiments with honey bees (Apis mellifera).
Animals as diverse as mammals, birds and insects use odors to find mates, hosts and food sources. This is a difficult task because natural odors occur in complex turbulent air plumes and the relevant target odors intermingle with plumes of multiple background odors stemming from a variety of natural and anthropogenic sources (vegetation, exhaust fumes, etc.).
It is essential for animals to be able to separate target from background odors. We hypothesize that animals may use temporal information from natural odor fluctuations to achieve odor-background segregation, as odorants from the same source fluctuate together in synchrony, while odorants from different sources do not.
Behavioral studies have shown that insects can indeed use fast temporal stimulus cues but it is not yet known how the brain accomplishes the difficult task of odor-background segregation. In this project we will investigate the neural mechanisms of odor-background segregation using the honey bee as model organism.
Honey bees visit many different flower species for nectar and pollen. However, over a series of trips an individual forager bee only visits a single flower species on which it has found nectar previously, a phenomenon called floral constancy. To localizes the target flowers in a natural environment bees use several cues, including odors. As the bee is challenged by multiple background odors in every flower patch, bees must be equipped with efficient mechanisms of odor-background segregation.
Noriyasu Ando*, Shuhei Emoto, Ryohei Kanzaki (2016) Insect-controlled robot: a mobile robot platform to evaluate odor-tracking capability of an insect. Journal of Visualized Experiments, 118 e54802. doi: 10.3791/54802
Noriyasu Ando*, Ryohei Kanzaki (2017) Using insects to drive mobile robots—hybrid robots bridge the gap between biological and artificial systems. Arthropod Structure and Development. in press. doi: 10.1016/j.asd.2017.02.003
Raiser Georg, Galizia C Giovanni, Szyszka Paul (2016) A high-bandwidth dual-channel olfactory stimulator for studying temporal sensitivity of olfactory processing Chem Senses 42(2):141-151. doi: 10.1093/chemse/bjw114
Ho Ka Chan, Yang D-P, Zhou C and Thomas Nowotny (2016) Burst Firing Enhances Neural Output Correlation. Front. Comput. Neurosci. 10:42. doi: 10.3389/fncom.2016.00042
Irina Sinakevitch, George R. Bjorklund, Jason M. Newbern, Richard C. Gerkin, Brian H. Smith (2017) Comparative study of chemical neuroanatomy of the olfactory neuropil in mouse, honey bee, and human. Biological Cybernetics doi: 10.1007/s00422-017-0728-8
, Mixtures are more salient stimuli in olfaction, bioRxiv (2017) http://www.biorxiv.org/content/early/2017/07/13/163238
The project is a collaboration between:-